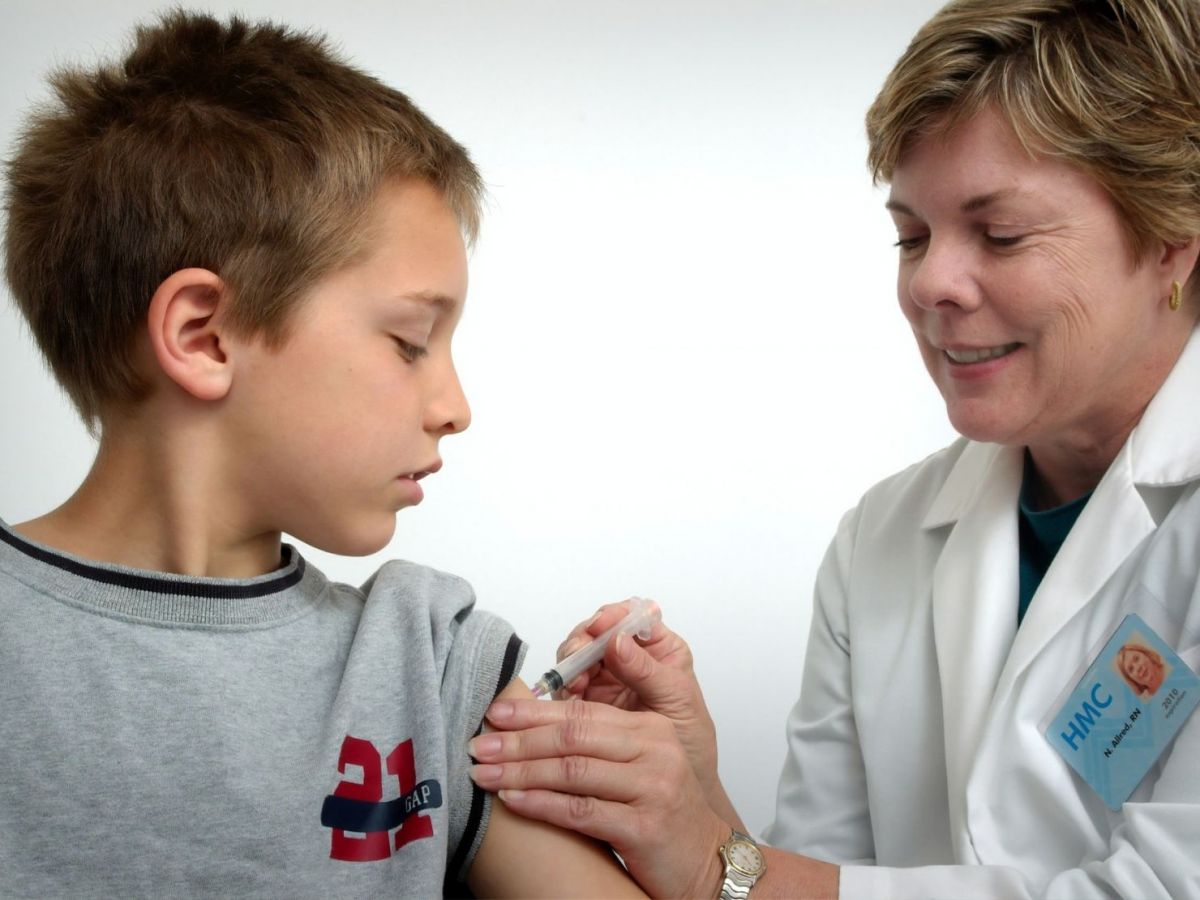
“The EMA Committee for Medicinal Products for Human Use has recommended granting an indication extension for the Comirnaty vaccine [brand name for the vaccine from the BioNTech/Pfizer pharmaceutical consortium] to include use in children aged 5-11 years,” says the European regulator in a statement. The vaccine was already in use from the age of 12 onwards.
The EMA explains that for children aged 5 to 11 years, the dose of Comirnaty “will be less than that used in people aged 12 and over”, but “as in the older age group, it is given as two injections in the forearm muscles, three weeks apart”.
This is the first vaccine approved in the EU for children in this age group, at a time when there is an increase in cases for these ages and when the United States is already administering it.
Currently, the Comirnaty vaccine is authorised from the age of 12, after being approved for the first time in December 2020 for adults in the EU.
In today's note, the EU regulator notes that “a main study in children aged 5 to 11 years showed that the immune response to Comirnaty given at a lower dose (10 µg) in this age group was comparable to that seen with the dose highest (30 µg) in children aged 16 to 25 years, as measured by the level of antibodies against SARS-CoV-2”.
“The effectiveness of Comirnaty was calculated based on a clinical trial carried out in almost 2,000 children aged 5 to 11 years who had no signs of previous infection”, adds the European agency, noting that “these children received either the vaccine or a placebo”.
According to the EMA, “of the 1305 children who received the vaccine, three developed covid-19 compared with 16 of the 663 children who received the placebo, meaning that, in this study, the vaccine was 90.7% effective in preventing the disease covid-19 symptomatic, although the actual rate could range between 67.7% and 98.3%”.
Side effects in children aged 5 to 11 years are similar to those in people aged 12 years and over, including pain, redness and swelling at the injection site, tiredness, muscle aches and chills, which are mild or moderate and improve within a few days after vaccination.
For all these reasons, the EMA Committee for Medicinal Products for Human Use “concluded that the benefits of Comirnaty in children aged 5 to 11 years outweigh the risks, particularly in those with conditions that increase the risk of covid-19”, the regulator said.
On Tuesday, the Portuguese Society of Pediatrics considered that vaccines against covid-19 are safe in the age group from 5 to 11 years old, but argued that the decision to vaccinate should take into account other data, such as the prevalence of the infection in children.